Research on sodium-ion batteries aims to reduce reliance on rare elements and cut costs, enhancing battery performance for broader applications. However, fast charging introduces mechanical stresses that compromise the battery’s structural integrity and longevity.
Simulations of microstructures demonstrate a significant impact of elastic deformation on the charging characteristics of layered oxides employed as cathodes in sodium-ion batteries.
Research is focused on enhancing the performance, longevity, and affordability of new battery materials. Efforts are also being made to decrease the use of scarce and toxic elements like lithium and cobalt. In this context, sodium-ion batteries are emerging as a promising alternative. They operate on principles akin to lithium-ion batteries but are made from raw materials that are readily available in Europe.
And they are suitable for both stationary and mobile applications. “Layered oxides, such as sodium-nickel-manganese oxides, are highly promising cathode materials,” says Dr. Simon Daubner, Group Leader at the Institute for Applied Materials – Microstructure Modeling and Simulation (IAM-MMS) of KIT and corresponding author of the study. Within the POLiS (stands for Post Lithium Storage) Cluster of Excellence, he investigates sodium-ion technology.
Fast Charging Creates Mechanical Stress
However, cathode materials of this type have a problem. Sodium-nickel-manganese oxides change their crystal structure depending on how much sodium is stored. If the material is charged slowly, everything proceeds in a well-ordered way. “Sodium leaves the material Layer by layer, just like cars leaving a carpark story by story,” Daubner explains. “But when charging is quick, sodium is extracted from all sides.” This results in mechanical stress that may damage the material permanently.
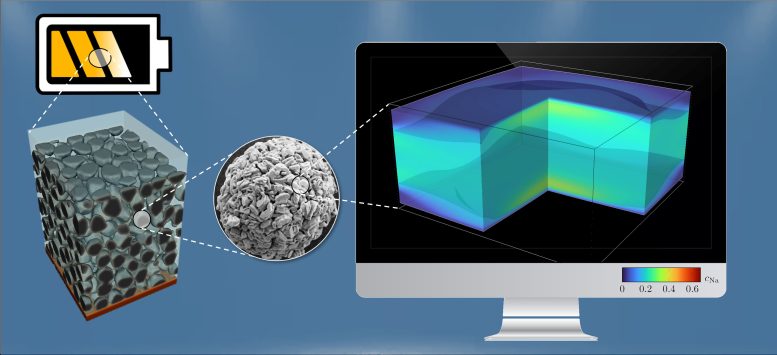
Section of a cathode layer (about 100 micrometers, left) consisting of spherical particles (of about ten micrometers in diameter, center) and simulation (right) of the sodium fraction in a sodium-nickel-manganese oxide crystal. Credit: Simon Daubner, KIT
Researchers from the Institute of Nanotechnology (INT) and IAM-MMS of KIT, together with scientists from Ulm University and the Center for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW), recently carried out simulations to clarify the situation. They report in npj Computational Materials, a journal of the Nature portfolio.
Experiments Confirm Simulation Results
“Computer models can describe various length scales, from the arrangement of atoms in electrode materials to their microstructure to the cell as the functional unit of any battery,” Daubner says. To study the NaXNi1/3Mn2/3O2 layered oxide, microstructured models were combined with slow charge and discharge experiments. The material was found to exhibit several degradation mechanisms causing a loss of capacity. For this reason, it is not yet suited for commercial applications. A change in the crystal structure results in an elastic deformation. The crystal shrinks, which may cause cracking and capacity reduction. INT and IAM-MMS simulations show that this mechanical influence decisively determines the time needed for charging the material. Experimental studies at ZSW confirm these results.
The findings of the study can be transferred partly to other layered oxides. “Now, we understand basic processes and can work on the development of battery materials that are long-lasting and can be charged as quickly as possible,” Daubner summarizes. This could lead to the widespread use of sodium-ion batteries in five to ten years’ time.
Reference: “Combined study of phase transitions in the P2-type NaXNi1/3Mn2/3O2 cathode material: experimental, ab-initio and multiphase-field results” by Simon Daubner, Manuel Dillenz, Lukas Fridolin Pfeiffer, Cornelius Gauckler, Maxim Rosin, Nora Burgard, Jan Martin, Peter Axmann, Mohsen Sotoudeh, Axel Groß, Daniel Schneider and Britta Nestler, 18 April 2024, npj Computational Materials.
DOI: 10.1038/s41524-024-01258-x
