
Scientists are exploring hydrogen as a clean energy source to combat climate change, focusing on its production through water electrolysis and use in fuel cells for transportation, aligning with the goal of net-zero carbon emissions by 2050.
What Is Hydrogen Energy?
As the effects of climate change take hold, our planet faces record heat waves, unprecedented storms, historic droughts, and wildfires. Scientists have linked these events to greenhouse gases like carbon dioxide in the atmosphere, much of which is produced by human activity.
But what if, instead of releasing harmful greenhouse gases into the environment, our airplanes and cars could run on fuel produced from water, using electricity from the sun or wind? What if this renewable fuel could provide backup power to the electric grid and be purchased from fueling stations across the nation?
[embedded content]
In this Science 101 video, scientists Debolina Dasgupta and Nancy Kariuki describe the science, technology, and applications of hydrogen energy. Hydrogen is the simplest chemical element, or type of <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{"attribute":"data-cmtooltip", "format":"html"}]” tabindex=”0″ role=”link”>atom, and an abundance of hydrogen exists within the water on our planet. It is naturally renewed by the water cycle, and when used as fuel, it releases no harmful emissions. For these reasons, hydrogen could play a major role in fostering a cleaner environment and reducing greenhouse gas emissions in sectors ranging from transportation to the grid. Scientists at the U.S. Department of Energy’s Argonne National Laboratory are leveraging world-class facilities and expertise to lower the cost of hydrogen production and develop affordable fuel cells for hydrogen-powered vehicles. They’re also assessing methods of hydrogen production, transport, storage and use to minimize greenhouse gas emissions.
Scientists are working to make this vision a reality using the energy within hydrogen, which promises to play a major role in fostering a cleaner environment and achieving the U.S. goal to attain net-zero carbon emissions by 2050 — in other words, removing carbon from the atmosphere at the same rate it is emitted.
Hydrogen is the simplest chemical element, or type of atom. It consists of just one proton and one electron. It is also the most abundant element, making up around 75% of the known matter in the universe. Vast amounts of hydrogen exist in water and living things.
An abundance of hydrogen exists within the water on our planet, and it is naturally renewed by the water cycle. When used as fuel, it releases no carbon emissions, making it a promising clean energy source. Credit: Argonne National Laboratory
The hydrogen molecule, consisting of two hydrogen atoms, can be used to produce carbon-free energy. Hydrogen molecules carry a lot of energy; a pound of hydrogen contains almost three times the energy of a pound of gasoline or diesel.
However, hydrogen molecules are not abundant on Earth, making up less than 0.0001% of our atmosphere. Because of this, hydrogen must be produced from other substances that contain it. The most common way to produce hydrogen that doesn’t use fossil fuels is to split water (H2O) into hydrogen (H2) and oxygen (O2) using electricity. This process, called water electrolysis, is a promising option for carbon-free hydrogen production since the electricity can be sourced from nuclear or renewable energy, such as wind and solar. Scientists and engineers are working to improve and lower the cost of hydrogen produced by water electrolysis.
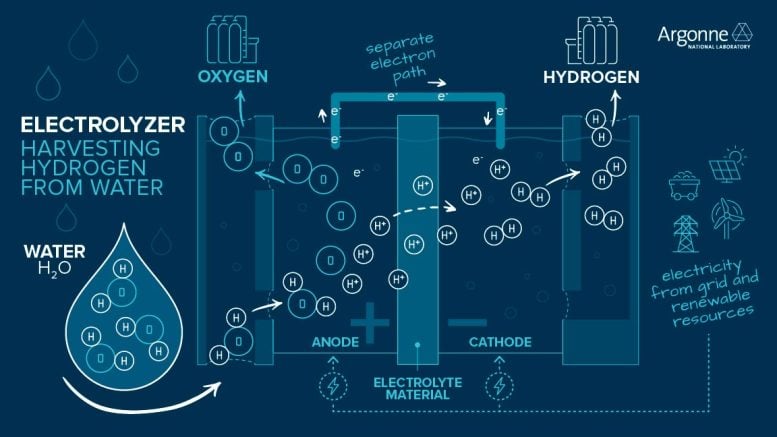
In electrolysis, water splits at the anode to form oxygen, hydrogen ions, and electrons. An electrolyte material allows hydrogen ions through, but forces electrons to flow separately to the cathode, where the two recombine to form hydrogen gas for use as fuel. Credit: Argonne National Laboratory
They are also developing methods that convert solar energy and water directly to hydrogen by harnessing and mimicking biological processes like <span class="glossaryLink" aria-describedby="tt" data-cmtooltip="
” data-gt-translate-attributes=”[{"attribute":"data-cmtooltip", "format":"html"}]” tabindex=”0″ role=”link”>photosynthesis.
There are several ways to use hydrogen for energy once it is produced. The most prominent is in fuel cells, which convert the chemical energy stored in hydrogen and oxygen into electricity. Unlike with gasoline-fueled engines, there are no harmful emissions like carbon dioxide. And unlike with batteries, fuel cell systems don’t require lengthy downtimes for recharging. They are refueled like gasoline-fueled engines, but with hydrogen.
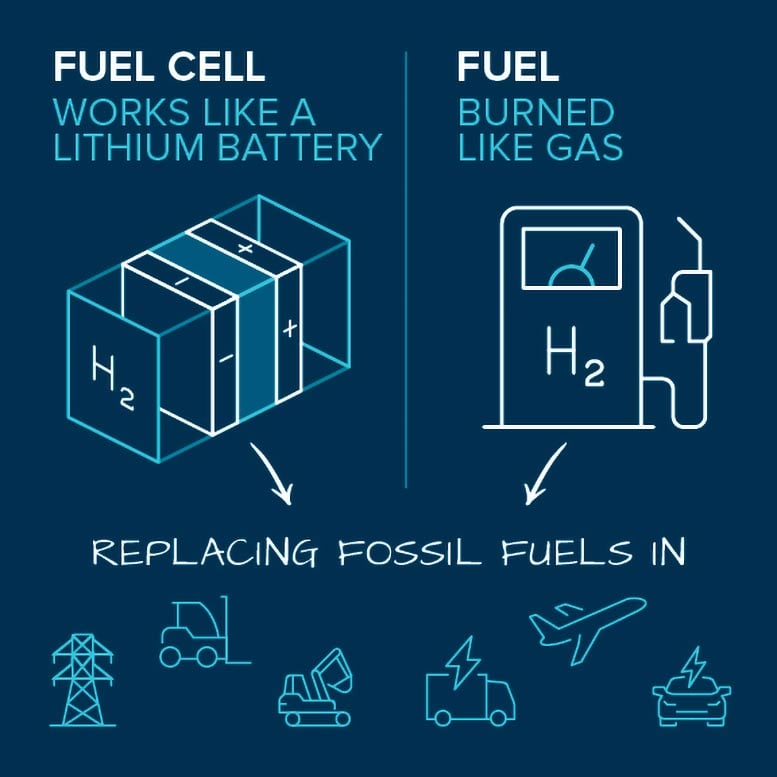
Hydrogen can be used in fuel cells or burned as fuel in engines. Scientists and engineers are working to improve these technologies, which could replace the use of fossil fuels in transportation and the grid. Credit: Argonne National Laboratory
A type of hydrogen fuel cell being developed for cars, trucks, forklifts, buses, ships, and trains splits hydrogen molecules into electrons and protons. The electrons are forced to flow through an electric circuit, creating a supply of usable electricity. Meanwhile, the protons are able to pass through a membrane, ultimately recombining with the electrons and reacting with oxygen molecules from the air to produce water, the only emission.
Scientists at the U.S. Department of Energy’s Argonne National Laboratory are leveraging world-class facilities and expertise to advance hydrogen science and technology. Our researchers are lowering the cost of hydrogen production, developing affordable fuel cells for hydrogen-powered vehicles. They’re also assessing methods of hydrogen production, transport, use, and storage to minimize greenhouse gas emissions.
